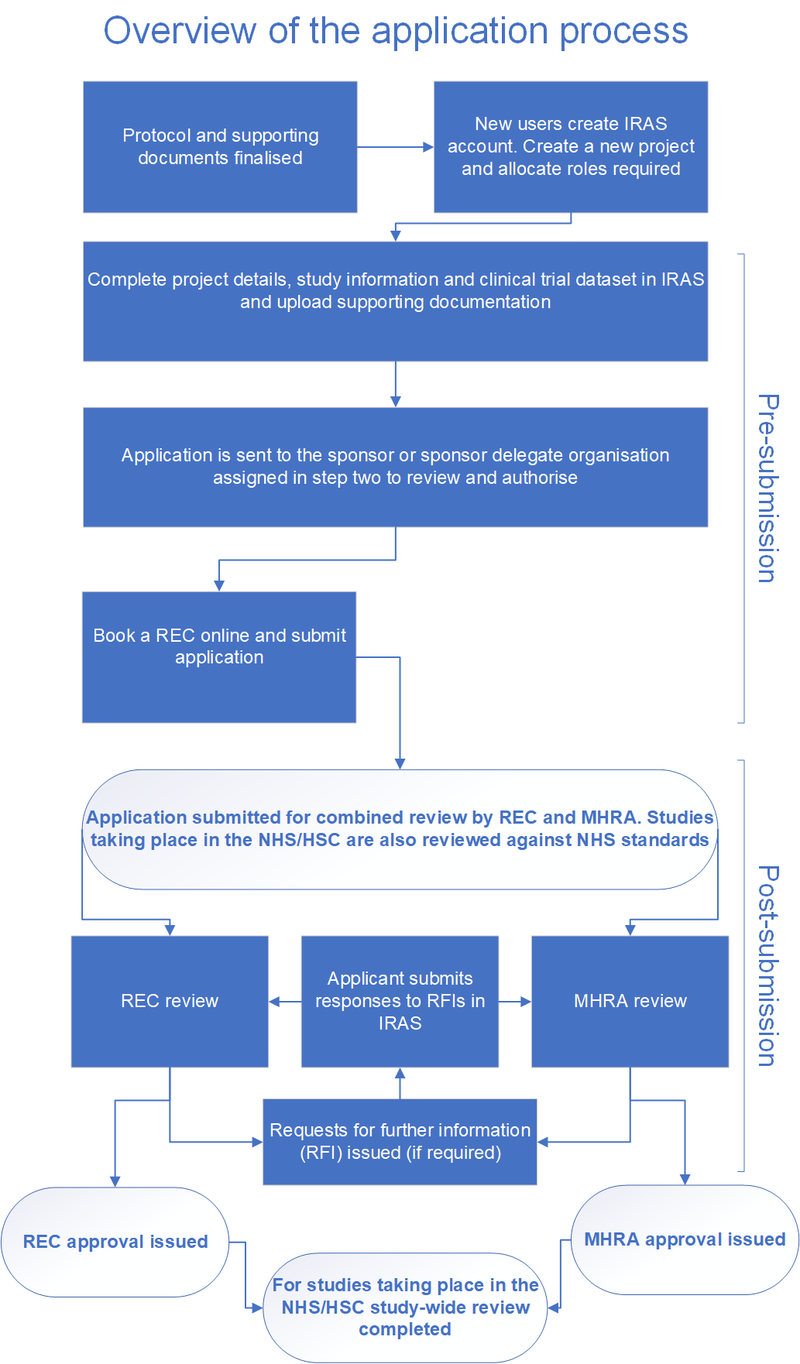
Detailed guidance on the application format and documentation to be submitted in an application for an Ethics Committee opinion

Federal Register :: Electronic Submission of Applications for Orders Under the Advisers Act and the Investment Company Act, Confidential Treatment Requests for Filings on Form 13F, and Form ADV-NR; Amendments to Form