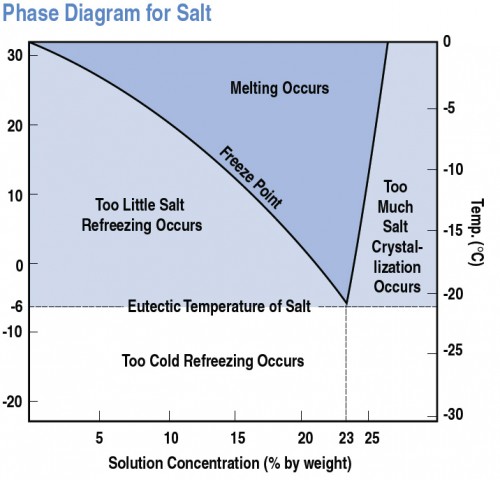
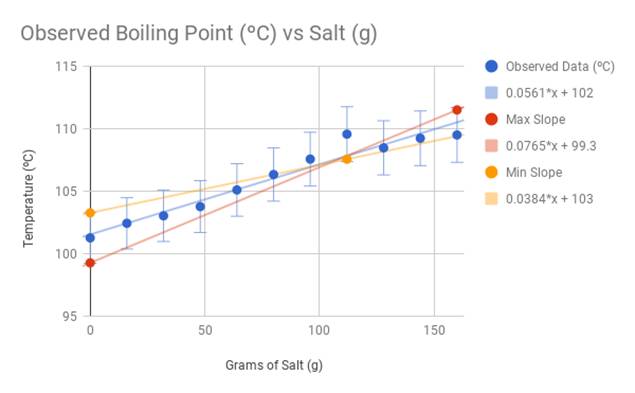
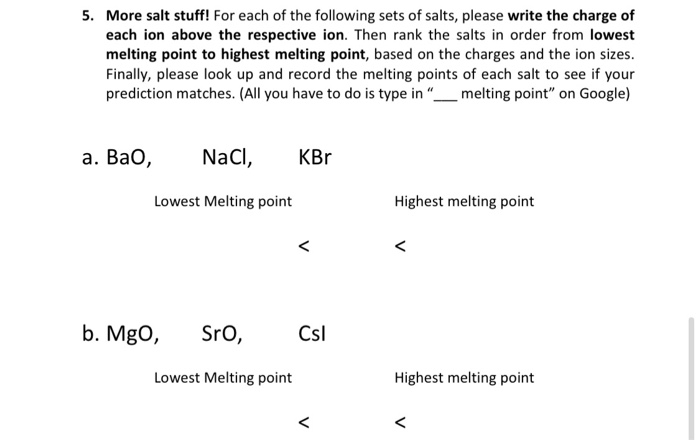
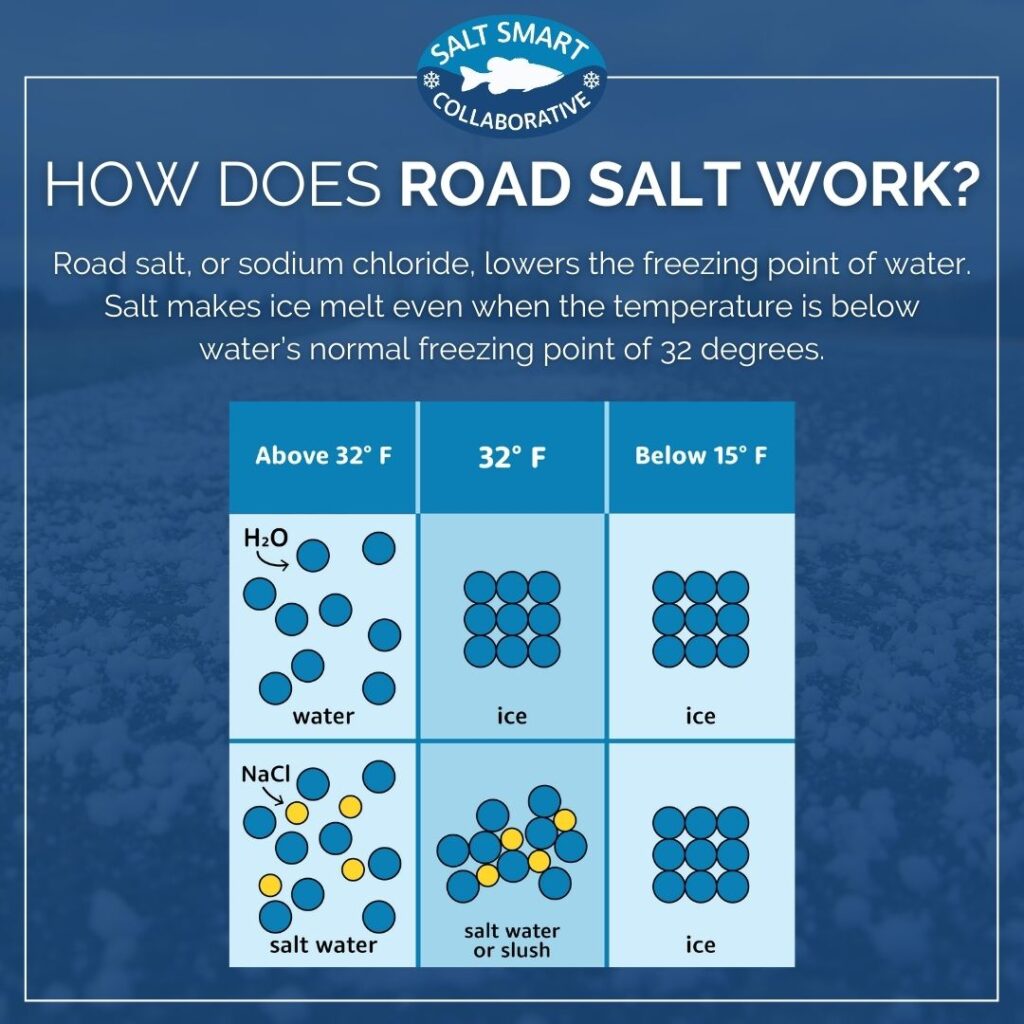
What happens to the melting point temperature of ice if you add common salt to it? JUSTIFY - Brainly.in

What has the lowest melting point? a. Sand, SiO2 b. Salt, NaCl C. Copper Chloride, CaCl2 d. Sugar, - brainly.com
Henna is investigating the melting point of different salt solutions. She makes a salt solution using 10 mL of water with a known mass of NaCl salt. She puts the salt solution

Understand how salt affects the freezing and melting points of water; the Heating Curve of Water Diagram | Quizlet

Table 1 from Aqueous solubility of organic salts. Investigating trends in a systematic series of 51 crystalline salt forms of methylephedrine | Semantic Scholar
![PDF] Melting points of binary and ternary eutectic chloride salts : MD simulations on LiCl-NaCl-KCl and its binary constituents | Semantic Scholar PDF] Melting points of binary and ternary eutectic chloride salts : MD simulations on LiCl-NaCl-KCl and its binary constituents | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6b24b4fe1a2ab72c5debe546b95fda5fd0112aaa/14-Table2.1-1.png)